Quality management with Doxis: Your advantages
Brochure

Quality management made easy
At the LWL clinics, quality takes top priority. With Doxis, the Regional Association of Westphalia-Lippe (LWL) fulfills all of the German KTQ quality requirements for healthcare. Read on to find out how the digital hospital manual takes the hassle out of regular re-certifications in this sector.
READ NOWDigital QM manual
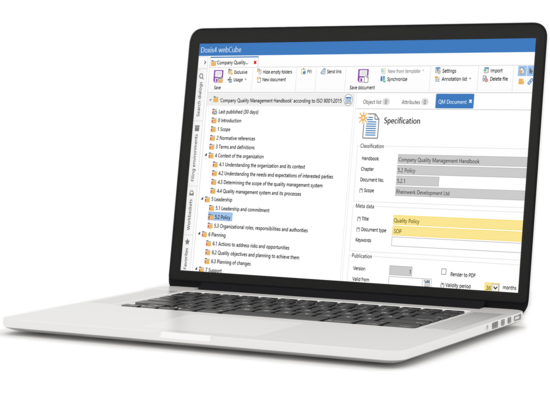
Map ISO structures quickly & easily with the Doxis QM Manual.
The web-based Doxis QM Manual can be viewed and modified from any location. Doxis comes already equipped with an ISO-compliant structure that can be adapted to your internal compliance structures at any time. Right out of the box, your QM manual is compliant with:
- ISO 9001:2015 – minimum requirements of a QM system
- ISO 14001:2015 – environmental management systems
- ISO 27001 – basic IT protection
- ISO 13485:2016 – medical devices
Integrated editing system
From work and procedural instructions to process descriptions and checklists, the integrated editing system of the Doxis QM manual helps you to create and assign your QM documents free from error.
Brochure

Quality management meets simplicity — with Doxis
Quality targets, guidelines and procedures: With Doxis you make sure that employees across the entire company stay completely up to date on all these aspects. Read on to find out how the Doxis QM Manual supports your quality management systems.
READ NOWPublication & confirmation
With Doxis, employees stay verifiably informed about QM guidelines.
Which guidelines are new? Have any process descriptions changed? Doxis automatically updates employees about changes in the QM Manual. Everyone can access the manual directly and conveniently online, including via the integrated intranet or employee portal. Employees simply acknowledge the changes directly with just one click in the Doxis QM Manual. Doxis documents this, so not only you can be sure that all employees are aware of the latest QM guidelines, but you also have the records to prove it.
How can we help you?
+49 (0) 30 498582-0Your message has reached us!
We appreciate your interest and will get back to you shortly.


